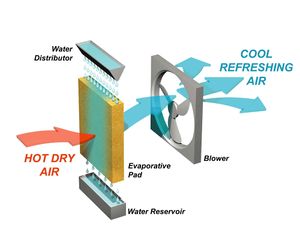
Evaporative Coolers
An Evaporative Cooler is a device that cools air through the evaporation of water. Evaporative cooling differs from typical air conditioning systems which use vapor-compression or absorption refrigeration cycles. Evaporative cooling works by employing water's large enthalpy of vaporization. The temperature of dry air can be dropped significantly through the phase transition of liquid water to water vapor (evaporation), which can cool air using much less energy than refrigeration. In extremely dry climates, evaporative cooling of air has the added benefit of conditioning the air with more moisture for the comfort of building occupants. Unlike closed-cycle refrigeration, evaporative cooling requires a water source, and must continually consume water to operate.
Working principle
Evaporative cooling is a physical phenomenon in which evaporation of a liquid, typically into surrounding air, cools an object or a liquid in contact with it. Latent heat, the amount of heat that is needed to evaporate the liquid, is drawn from the air. When considering water evaporating into air, the wet-bulb temperature which takes both temperature and humidity into account, as compared to the actual air temperature , is a measure of the potential for evaporative cooling. The greater the difference between the two temperatures, the greater the evaporative cooling effect. When the temperatures are the same, no net evaporation of water in air occurs, thus there is no cooling effect. The wet-bulb temperature is essentially the lowest temperature which can be attained by evaporative cooling at a given temperature and humidity.
Air washers and wet Cooling Towers use the same principles as evaporative coolers but are designed for purposes other than directly cooling the air inside a building. For example, an evaporative cooler may be designed to cool the coils of a large air conditioning or refrigeration system to increase its efficiency.